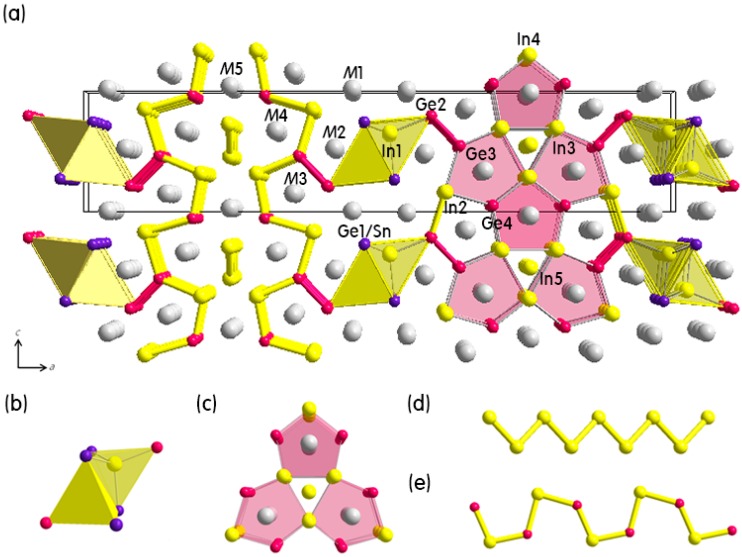
Three quinary polar intermetallic compounds in the (Eu(1-x)Ca(x))9In8(Ge(1-y)Sn(y))8 (x = 0.66, y = 0.03) and the (Eu(1-x)Ca(x))3In(Ge(3-y)Sn(1+y)) (x = 0.66, 0.68; y = 0.13, 0.27) phases have been synthesized utilizing the molten In-metal flux technique, and the crystal constructions are characterised by powder and single-crystal X-ray diffractions.
Two orthorhombic structural sorts may be seen as an meeting of polyanionic frameworks consisting of the In(Ge/Sn)four tetrahedral chains, the bridging Ge2 dimers, both the annulene-like “12-membered rings” for the (Eu(1-x)Ca(x))9In8(Ge(1-y)Sn(y))Eight sequence or the cis-trans Ge/Sn-chains for the (Eu(1-x)Ca(x))3In(Ge(3-y)Sn(1+y)) sequence, and a number of Eu/Ca-mixed cations.
The most noticeable distinction between two structural sorts is the quantity and the location of the Sn-substitution for Ge: solely a partial substitution (11%) happens at the In(Ge/Sn)four tetrahedron in the (Eu(1-x)Ca(x))9In8(Ge(1-y)Sn(y))Eight sequence, whereas each a whole and a partial substitution (as much as 27%) are noticed, respectively, at the cis-trans Ge/Sn-chain and at the In(Ge/Sn)four tetrahedron in the (Eu(1-x)Ca(x))3In(Ge(3-y)Sn(1+y)) sequence.
A sequence of tight-binding linear muffin-tin orbital calculations is performed to grasp total digital constructions and chemical bonding amongst parts. Magnetic susceptibility measurement signifies a ferromagnetic ordering of Eu atoms beneath 5 Okay for Eu1.02(1)Ca1.98InGe2.87(1)Sn1.13.
Characterization of construction, physico-chemical properties and diffusion conduct of Ca-Alginate gel beads ready by completely different gelation strategies.
Ca-Alginate beads had been ready with both exterior or inside calcium sources by dripping approach. It was discovered that beads synthesized with inside calcium supply had a looser construction and greater pore measurement than these produced with exterior calcium supply.
Consequently, a quicker diffusion charge of Vitamin B12 (VB12) inside the beads with an inside calcium supply was noticed.
Furthermore, the focus of calcium ion, ionic power and pH of the exterior gel beads formation resolution had been investigated. Results confirmed that (a) the focus of the calcium ion was discovered to be the figuring out issue in the gel formation phenomenon; (b) the weight and quantity losses are in impact on account of water elimination; (c) NaCl acts as a competitor with calcium and a display in the electrostatic repulsion; and (d) the pH controls the gel formation course of by regulating the dissociation of alginate and the complexation of the calcium cations.
These outcomes are keys to understanding the conduct and efficiency of beads in their utilization medium.
